Background and Objective:Mastocytosis patients suffer from a clonal increase in mast cells which produce and release excessive amounts of histamine even under stable conditions (Keyzer et al. 1983). Unfortunately, symptom control with new kinase inhibitors is variable and modest at best. Mast cell activation syndrome may further enhance histamine release which can result in recurrent shocks (Böhm et al. 2019), or severe, protracted anaphylaxis refractory to epinephrine (Grafeneder et al. 2022). Previously we demonstrated that recombinant human diamine oxidase (rhDAO) fully degrades 100-fold elevated histamine concentrations in plasma samples from mastocytosis patients. In vivo rhDAO, with a mutated heparin binding motif (mHBM), was able to digest massively increased histamine levels, thereby improving indirect measures of shock such as a temperature drop in mice (Karer et al. 2022). As mice are insensitive to histamine (intravenous LD50 of 385mg/kg in mice compared with 0.18mg/kg in guinea pigs), this unfortunately limits their translational value. To assess the therapeutic potential of rhDAO_mHBM, we tested its efficacy for the prevention and treatment of histamine induced shock in guinea pigs, the only histamine sensitive rodent species.
Methods:Guinea pigs received continuous intravenous infusions of 8 µg/kg/min histamine to model its release from activated circulating basophils or subcutaneous injections of 1mg/kg histamine to mimic peripheral histamine release by mast cells. We used intramuscular injections of 5µg/kg adrenaline as a benchmark, because it is recommended in guidelines for the treatment of anaphylaxis, although its efficacy had never been proven. Infusions of rhDAO_mHBM were given either prophylactically before- or therapeutically after - symptom onset. Mean arterial pressure was measured intra-arterially and rhDAO_mHBM levels were quantified by enzyme immunoassay and histamine concentrations by a liquid chromatography -tandem mass spectrometry (LC-MS/MS) method. The primary outcome variable was prevention of shock as defined by a >=30% drop in mean arterial blood pressure.
Results:Guinea pigs pretreated with 4 mg/kg rhDAO_mHBM showed lower mean heart rate (p<0.01) and higher body core temperatures at the end of the intravenous histamine infusions compared to controls (p<0.05). Histamine infusions increased median urinary excretion of histamine and N-methyl-histamine 8.6- and 4.0-fold (p<0.05), respectively. Pretreatment with 4 mg/kg rhDAO_mHBM reduced urinary histamine (p<0.01) and N-methyl-histamine (p<0.01) concentrations compared with controls.
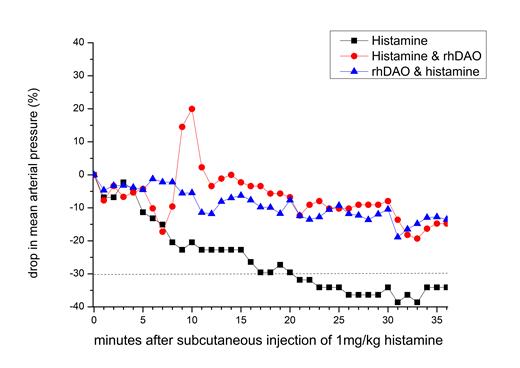
Subcutaneous injections of 1 mg/kg histamine induced shock within 20 minutes in all control animals, and the experiments had to be terminated prematurely in several animals. Intramuscular injection of 5mcg/kg adrenaline (similar to human adrenaline auto-injector doses) after symptom onset was unable to prevent shock, confirming its lack of efficacy demonstrated previously in an anaphylaxis model in dogs (Mink et al. 2004). In contrast, prophylactic infusions of 4-8mg/kg rhDAO_mHBM prevented hypotensive shock in all treated animals (p<0.01). After an initial histamine induced drop in blood pressure, 8mg/kg rhDAO_mHBM restored blood pressure and prevented shock in all animals when injected at the time of symptom occurrence (p<0.01 Chi2 test). Figure 1 demonstrates the median drop in blood pressure and development of shock (indicated by the dashed line) by subcutaneous injection of 1mg/kg histamine (black squares), and the prevention of shock by rhDAO-mHBM given before (blue up triangles) or after (red circles) histamine injections.
Conclusion: This is the first demonstration of the beneficial effects of rhDAO_mHBM in a histamine-sensitive species. rhDAO_mHBM is a novel therapeutic approach targeting histamine excess which effectively prevented shock, in contrast to intramuscular epinephrine. As the mechanism of action is not species specific, these findings can be translated from guinea pigs to humans: it will help to select optimal dosing of rhDAO_mHBM during clinical development for the prophylaxis in - and treatment of - mastocytosis patients suffering from histamine induced symptoms or mast cell activation syndrome, or patients with anaphylaxis induced by asparaginase, plasma products or other medications.
Disclosures
Jilma:Sanofi: Consultancy, Honoraria, Speakers Bureau; Guardian Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal